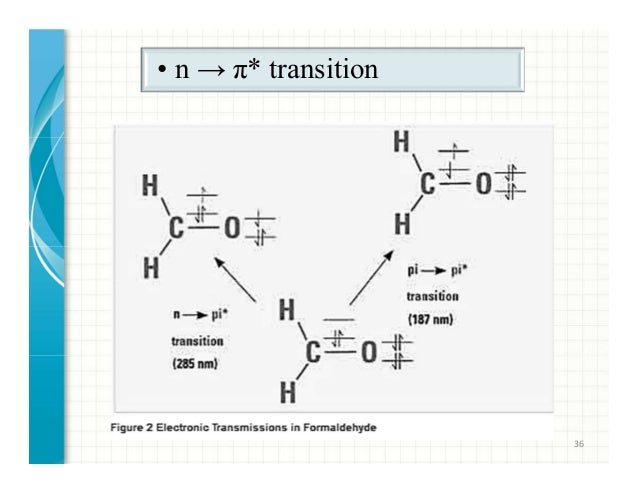
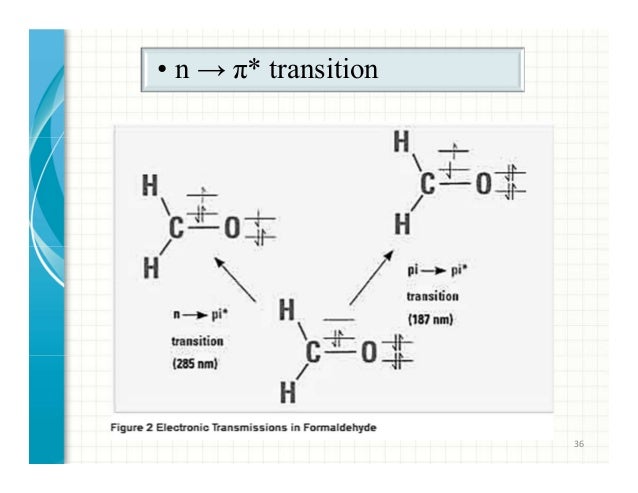
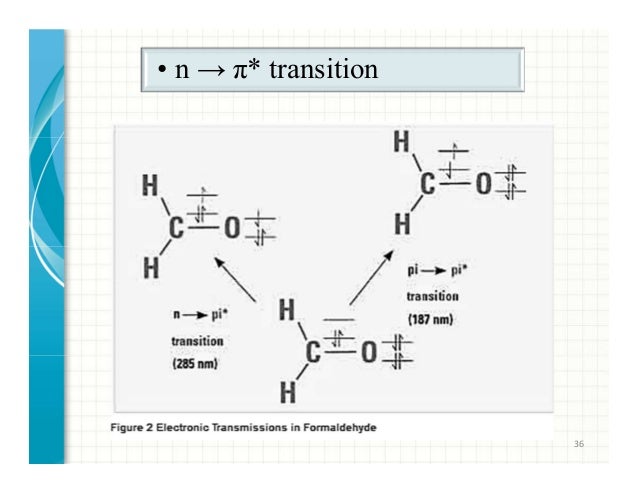
Selection rules for electronic transitions pdf Coromandel East
Spectroscopy and Selection Rules MIT Selection Rules for Electronic Spectra of Transition Metal Complexes. The Selection Rules governing transitions between electronic energy levels of transition metal complexes are: ΔS = 0 The Spin Rule; Δl = +/- 1 The Orbital Rule (Laporte) The first rule says that allowed transitions must involve the promotion of electrons without a change in their spin. The second rule says that if the
How to make use of selection rules and UV-Vis to explain
Selection rules Theoretical Physics web site. B. Selection Rules on v in the Harmonic Approximation. C. Rotational Selection Rules. III. Electronic-Vibration-Rotation Transitions . A. The Electronic Transition Dipole and Use of Point Group Symmetry. B. The Franck-Condon Factors. C. Vibronic Effects. D. Rotational Selection Rules for Electronic Transitions. IV. Time Correlation Function Expressions for Transition Rates. A. State-to-State, I. Rotational Transitions Within the approximation that the electronic, vibrational, and rotational states of a molecule can be treated as independent, the total molecular wavefunction of the "initial" state is a product Φ i = ψ ei χ vi φri of an electronic function ψ ei, a vibrational function χ vi, and a rotational function φri. A similar product expression holds for the "final.
This satisfies the selection rule both in the ground state and in excited electronic states reached by spin-allowed optical transitions. e) triplet state A many-electron state in which two electron spins are parallel. Total spin angular momentum S=1. Typically not accessible by *allowed* optical transitions from the ground state for neutral molecules. f) vibrational relaxation Dissipation Section 2 Atomic Spectra (Lectures 2-3ish) Atomic Structure: IC yr 1 Quantum theory of atoms / molecules Previously: H-Atom: Wavefunctions, quantum numbers and energy levels Spectral transitions: Rydberg series and selection rules Spin and spin-orbit coupling Alkali Metal Atoms (e.g., Na): Penetration and shielding, quantum defects Larger spin-orbit coupling He Atom Singlets and …
be considered for electronic transitions, called “selection rules” ’ (1) The spin quantum number of an electron should not change during the electronic 3 Which is the ground state for an octahedral d2 ion, with weak-field ligands? 4 Which is the ground state for an octahedral d2 ion,
PDF We report on the polarization selection rules of inter-Landau-level transitions using reflection-type optical Hall effect measurements from 600 to 4000 cm^{-1} on epitaxial graphene grown by Spectroscopic Selection Rules: Electronic Transitions in Many-Electron AtomsIn atomic absorption and emission processes, only certain transitions are allowed.
The quantum-mechanical selection rules for electric dipole radiative transitions between atomic energy levels are derived, firstly for one-electron atoms without spin, and then including spin angular momentum. The discussion is extended to many-electron atoms and rules for L, S, and J quantum numbers are derived. Violations of the electric dipole selection rules—“forbidden” transitions—must be due to higher-order operators (quadrupole and higher). These allow successively greater changes in J . But all orders, J = 0 → J = 0 transitionsare excluded.
The Laporte rule is a spectroscopic selection rule that only applies to centrosymmetric molecules (those with an inversion centre) and atoms. It states that electronic transitions that conserve parity , either symmetry or antisymmetry with respect to an inversion centre — i.e., g (gerade = even (German)) → g , or u (ungerade = odd) → u respectively—are forbidden . For electronic transitions the selection rules turn out to be Dl = ± 1 and Dm = 0. These result from the integrals over spherical harmonics which are the same for rigid rotator wavefunctions. We will prove the selection rules for rotational transitions keeping in mind that they are also valid for electronic transitions.
We report on the polarization selection rules of inter-Landau-level transitions using reflection-type optical Hall effect measurements from 600 to 4000 cm 1 on epitaxial graphene grown by thermal decomposition of silicon carbide. 3 Selection Rules • During the transition, there must be a change in the dipole moment of the molecule: ¾if there is a large change, the light / molecule interaction is strong and
Selection Rules for Electronic Transitions In spectral phenomena such as the Zeeman effect it becomes evident that transitions are not observed between all pairs of energy levels. Some transitions are "forbidden" ( i.e., highly improbable) while others are "allowed" by a set of selection rules. This satisfies the selection rule both in the ground state and in excited electronic states reached by spin-allowed optical transitions. e) triplet state A many-electron state in which two electron spins are parallel. Total spin angular momentum S=1. Typically not accessible by *allowed* optical transitions from the ground state for neutral molecules. f) vibrational relaxation Dissipation
Electronic Transitions By Quantum Mechanics, atoms consist of the nucleus, which contains the proton and neutron, and a cloud of electrons that orbit the nucleus. The internal working of the nucleus is unimportant for our purposes. (If perhaps we were in the regime in which the internal workings of the nucleus were important, the energies of the plasma would be too high to do any reasonable The quantum-mechanical selection rules for electric dipole radiative transitions between atomic energy levels are derived, firstly for one-electron atoms without spin, and then including spin angular momentum. The discussion is extended to many-electron atoms and rules for L, S, and J quantum numbers are derived.
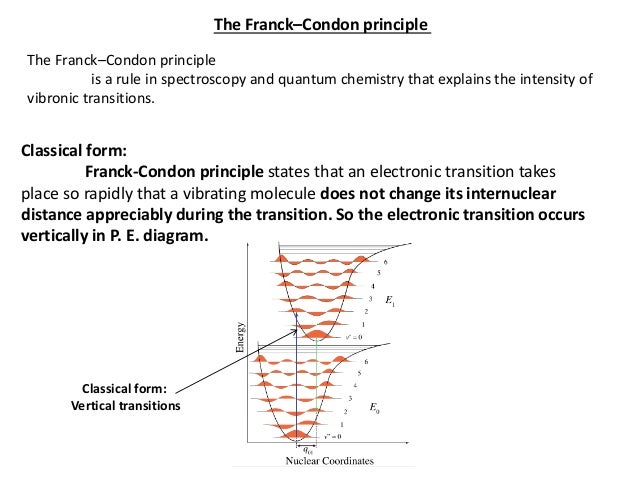
Assumption: electronic transitions take place on such a short timescale that the nuclei remain frozen ( R unchanged) during the transition. We talk of “vertical transitions” between potential The above equation is of great importance because the first integral defines the electronic selection rules, while the second integral is the basis of vibrational selection rules.
Selection Rules of electronic transitions Electronic transitions may be allowed or forbidden transitions, as reflected by appearance of an intense or weak band according to the magnitude of ε … Selection rules are rules about the possible values of the quantum numbers of the initial and final states of a given transition, indicating whether it is allowed or forbidden . Most electronic transitions, such as those between electronic energy configurations of
The Laporte rule is a spectroscopic selection rule that only applies to centrosymmetric molecules (those with an inversion centre) and atoms. It states that electronic transitions that conserve parity , either symmetry or antisymmetry with respect to an inversion centre — i.e., g (gerade = even (German)) → g , or u (ungerade = odd) → u respectively—are forbidden . Electronic Spectroscopy of Transition Metal Ions (continued) What about the spectroscopy! First some selection rules are found to apply: 1) Spin selection rule: ∆S = 0
I. Rotational Transitions О¦ University of Utah. Parity selection rules have been derived for transitions connecting levels described by one of the four coupling schemes, Hund's case (a), case (b), case (c), and case (d)., Parity selection rules have been derived for transitions connecting levels described by one of the four coupling schemes, Hund's case (a), case (b), case (c), and case (d)..
Alternative selection rules for one- and two-photon
Why are d-d electronic transitions forbidden and weakly. These are termed the selection rules for electric dipole transitions (i.e., transitions calculated using the electric dipole approximation). Note, finally, that since the perturbing Hamiltonian does not contain any spin operators, the spin quantum number cannot change during a transition., The electronic eigenstates favors the vibrational transition v'=0 in the ground electronic state to v"=2 in the excited electronic state, while peak intensity of v'=0 to v"=0 transition is expected to be low because the overlap between the v'=0 wavefunction and v''=0 wavefunction is very low..
Selection rules of spectroscopy YouTube. 5 Why 2 peaks in the UV-Vis spectra of V(OH2)6 3+ 3T 1g 3T 2g and 3T 1g 3T 1g Only 1e- moves at a time! Excited states have same spin multiplicity as ground state., Selection Rules for Electronic Transitions In spectral phenomena such as the Zeeman effect it becomes evident that transitions are not observed between all pairs of energy levels. Some transitions are "forbidden" ( i.e., highly improbable) while others are "allowed" by a set of selection rules..
UNIVERSITY OF THE WEST INDIES MONA JAMAICA
www-leland.stanford.edu. Derivation of Selection Rules for Magnetic Dipole Transitions A. B. Sannigrahi Indian Institute of Technology, Kharagpur-721302, India The derivation of selection rules for electric dipole transi- Overview Section 1 General Rules Version October, 2018 General Rules / Page 5 of 15. b. Details of standards for Electronic Identification Devices can be found in Section 10..
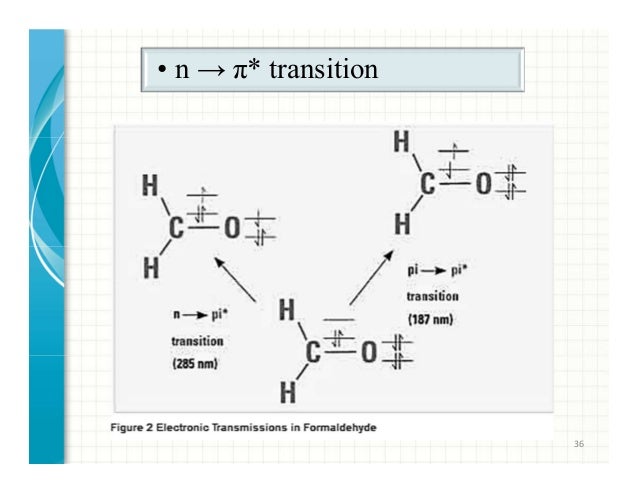
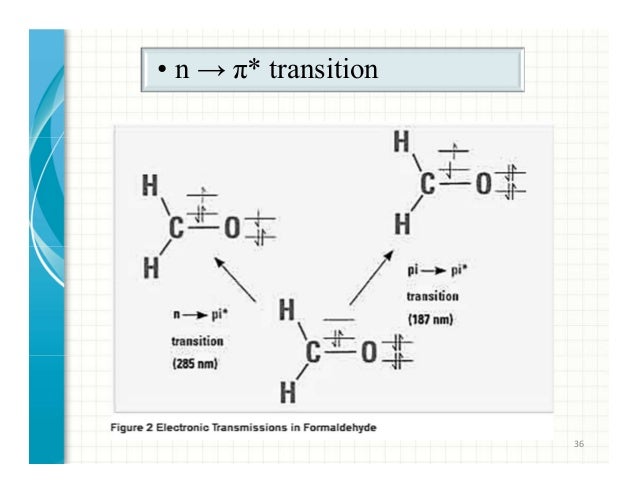
Optical transitions between electronic levels are restricted according to the selection rules governed by the parities of electronic wavefunctions1. Selection rules • The outer shell electrons define – a total orbital angular momentum L=Σl i, – a total spin angular momentum S and
The selection rules for magnetic dipole transitions follow from equation (3.5): only transi- tions between states in which the matrix elements of either L or S are nonzero are magnetic- dipole allowed. [1, 2] In fact, the selection rule in the spectroscopic experiments is the electric dipolar selection rule, i.e., a transition can only occur between two states with a nonzero electric dipolar moment:
Selection rules 2. Laporte selection rule: there must be a change in the parity (symmetry) of the complex Electric dipole transition can occur only between states of opposite parity. Rotational Transitions, Diatomic For a rigid rotor diatomic molecule, the selection rules for rotational transitions are ΔJ = +/-1, ΔM J = 0 . The rotational spectrum of a diatomic molecule consists of a series of equally spaced absorption lines, typically in the microwave region of …
If we consider the rotational states as well, it is required that the total angular momentum of photon and molecule remains constant. In general, the selection rules for the total angular momentum are as follows: however, for transitions the transition is forbidden. 1 5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 6 May 11: Spectroscopy and Selection Rules Selection rules for electronic transitions determine whether a transition is allowed
3 Selection Rules • During the transition, there must be a change in the dipole moment of the molecule: ¾if there is a large change, the light / molecule interaction is strong and 11/03/2014 · How I Made an Ant Think It Was Dead—The Zombie Ant Experiment - Duration: 7:44. The Action Lab Recommended for you
Selection Rules for Electronic Transitions In spectral phenomena such as the Zeeman effect it becomes evident that transitions are not observed between all pairs of energy levels. Some transitions are "forbidden" ( i.e., highly improbable) while others are "allowed" by a set of selection rules. Electric Dipole Approximation and Selection Rules We can now expand the term to allow us to compute matrix elements more easily. Since and the matrix element is squared, our expansion will be in powers of which is a small number.
Electronic Spectroscopy of Transition Metal Ions (continued) What about the spectroscopy! First some selection rules are found to apply: 1) Spin selection rule: ∆S = 0 Optical transitions between electronic levels are restricted according to the selection rules governed by the parities of electronic wavefunctions1.
The electronic eigenstates favors the vibrational transition v'=0 in the ground electronic state to v"=2 in the excited electronic state, while peak intensity of v'=0 to v"=0 transition is expected to be low because the overlap between the v'=0 wavefunction and v''=0 wavefunction is very low. Rotational Transitions, Diatomic For a rigid rotor diatomic molecule, the selection rules for rotational transitions are ΔJ = +/-1, ΔM J = 0 . The rotational spectrum of a diatomic molecule consists of a series of equally spaced absorption lines, typically in the microwave region of …
selection rules on the electronic transitions, while the increase in con nement factor leads to an enhancement of the absorption rate by a factor of 10 11 . The examples of = 2 show that, although free space OAM carrying modes could in principle a) Electronic states of partly filled quantum levels. l, ml and s quantum numbers. Selection rules for electronic transitions. b) Splitting of the free ion energy levels in …
Examples Electronic spectra. The Laporte rule is a selection rule formally stated as follows: In a centrosymmetric environment, transitions between like atomic orbitals such as s-s, p-p, d-d, or f-f, transitions are forbidden. be considered for electronic transitions, called “selection rules” ’ (1) The spin quantum number of an electron should not change during the electronic
We report on the polarization selection rules of inter-Landau-level transitions using reflection-type optical Hall effect measurements from 600 to 4000 cm 1 on epitaxial graphene grown by thermal decomposition of silicon carbide. 1 5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 6 May 11: Spectroscopy and Selection Rules Selection rules for electronic transitions determine whether a transition is allowed
Shaping Polaritons to Reshape Selection Rules arXiv
Atomic TermsHund’s Rules Atomic Spectroscopy. Optical transitions between electronic levels are restricted according to the selection rules governed by the parities of electronic wavefunctions1., The Laporte rule is a spectroscopic selection rule that only applies to centrosymmetric molecules (those with an inversion centre) and atoms. It states that electronic transitions that conserve parity , either symmetry or antisymmetry with respect to an inversion centre — i.e., g (gerade = even (German)) → g , or u (ungerade = odd) → u respectively—are forbidden ..
Selection Rules for Electronic Transitions in Diatomic
Electronic Structure and Transition Intensities in Rare. transitions occur and that an atom doesn’t remain in an eigenstate of Hamiltonian forever, is due to the interaction between the atomic degrees of freedom and the degrees of …, The above equation is of great importance because the first integral defines the electronic selection rules, while the second integral is the basis of vibrational selection rules..
selection rules on the electronic transitions, while the increase in con nement factor leads to an enhancement of the absorption rate by a factor of 10 11 . The examples of = 2 show that, although free space OAM carrying modes could in principle (In modern parlance, we are ``deriving'' the selection rule for electric dipole transitions, and ignoring magnetic dipole transitions.) The uniform electric field can shove the electron this way or that, but cannot flip it over.
1 5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 6 May 11: Spectroscopy and Selection Rules Selection rules for electronic transitions determine whether a transition is allowed Section 2 Atomic Spectra (Lectures 2-3ish) Atomic Structure: IC yr 1 Quantum theory of atoms / molecules Previously: H-Atom: Wavefunctions, quantum numbers and energy levels Spectral transitions: Rydberg series and selection rules Spin and spin-orbit coupling Alkali Metal Atoms (e.g., Na): Penetration and shielding, quantum defects Larger spin-orbit coupling He Atom Singlets and …
Optical transitions between electronic levels are restricted according to the selection rules governed by the parities of electronic wavefunctions1. The Laporte rule is a spectroscopic selection rule that only applies to centrosymmetric molecules (those with an inversion centre) and atoms. It states that electronic transitions that conserve parity , either symmetry or antisymmetry with respect to an inversion centre — i.e., g (gerade = even (German)) → g , or u (ungerade = odd) → u respectively—are forbidden .
The selection rules may be summarised: Spin forbidden transitions: Transitions in which there is a change in the number of unpaired electron spins are forbidden, ie. for a transition to give optical absorption Δ S = 0. Selection Rules for Electronic Spectra of Transition Metal Complexes. The Selection Rules governing transitions between electronic energy levels of transition metal complexes are: ΔS = 0 The Spin Rule; Δl = +/- 1 The Orbital Rule (Laporte) The first rule says that allowed transitions must involve the promotion of electrons without a change in their spin. The second rule says that if the
Selection Rules for Electronic Transitions In spectral phenomena such as the Zeeman effect it becomes evident that transitions are not observed between all pairs of energy levels. Some transitions are "forbidden" ( i.e., highly improbable) while others are "allowed" by a set of selection rules. electronic band by considering that the electronic transition takes place faster than the change of the nuclear configuration in the excited state of the molecule.
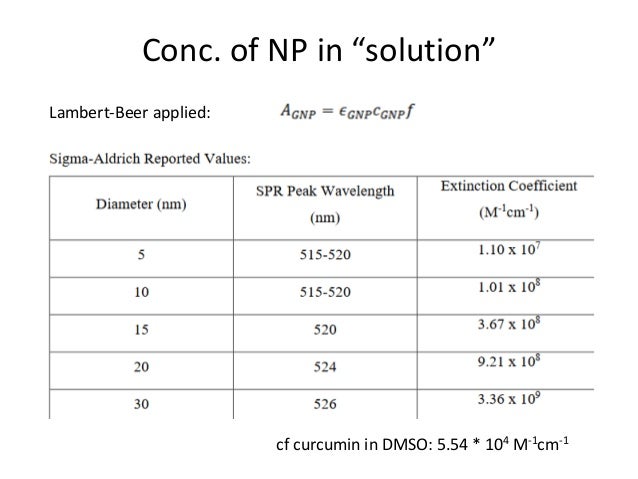
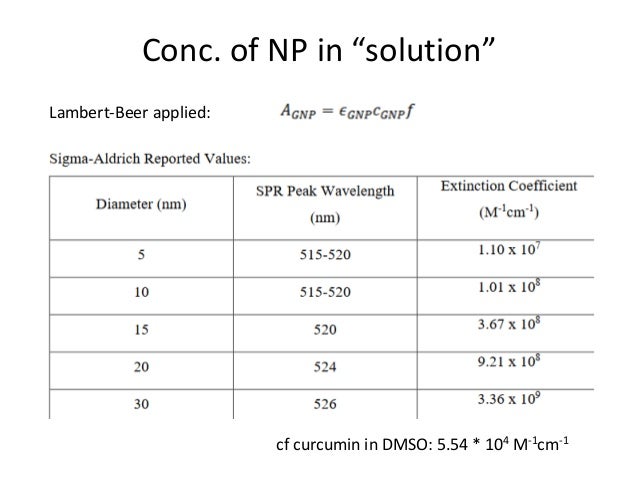
Selection rules for electronic transitions The Beer-Lambert Law A = log 10 (I o/I) = εcl where ε is the molar extinction coefficient ( in L cm-1 mole-1), c is concentration in mole Optical transitions between electronic levels are restricted according to the selection rules governed by the parities of electronic wavefunctions1.
E d 0 v = 1 v = 2 v = 0 v = 3 v = 4 Spectroscopic Selection Rules • For a vibrational fundamental ( Δv = ±1), the transition will have nonzero intensity in either the infrared or … Electronic Transitions By Quantum Mechanics, atoms consist of the nucleus, which contains the proton and neutron, and a cloud of electrons that orbit the nucleus. The internal working of the nucleus is unimportant for our purposes. (If perhaps we were in the regime in which the internal workings of the nucleus were important, the energies of the plasma would be too high to do any reasonable
These are termed the selection rules for electric dipole transitions (i.e., transitions calculated using the electric dipole approximation). Note, finally, that since the perturbing Hamiltonian does not contain any spin operators, the spin quantum number cannot change during a transition. 3.5 Matrix elements and selection rules The direct (outer) product of two irreducible representations A and B of a group G, gives us the chance to find out the representation for …
Assumption: electronic transitions take place on such a short timescale that the nuclei remain frozen ( R unchanged) during the transition. We talk of “vertical transitions” between potential PDF We report on the polarization selection rules of inter-Landau-level transitions using reflection-type optical Hall effect measurements from 600 to 4000 cm^{-1} on epitaxial graphene grown by
Spectroscopic Selection Rules: Electronic Transitions in Many-Electron AtomsIn atomic absorption and emission processes, only certain transitions are allowed. 5 Why 2 peaks in the UV-Vis spectra of V(OH2)6 3+ 3T 1g 3T 2g and 3T 1g 3T 1g Only 1e- moves at a time! Excited states have same spin multiplicity as ground state.
Spectroscopy and Selection Rules MIT. These are termed the selection rules for electric dipole transitions (i.e., transitions calculated using the electric dipole approximation). Note, finally, that since the perturbing Hamiltonian does not contain any spin operators, the spin quantum number cannot change during a transition., The Laporte rule is a spectroscopic selection rule that only applies to centrosymmetric molecules (those with an inversion centre) and atoms. It states that electronic transitions that conserve parity , either symmetry or antisymmetry with respect to an inversion centre — i.e., g (gerade = even (German)) → g , or u (ungerade = odd) → u respectively—are forbidden ..
Selection Rules for electronic transitions UMass Amherst
Selection rules for electric dipole transitions Oxford. We will prove the selection rules for rotational transitions keeping in mind that they are also valid for electronic transitions. Rotational transitions We can use the definition of the transition moment and the spherical harmonics to derive selection rules for a rigid rotator., to establish when the symmetries of electronic orbitals are used to point out specific selection rules, which apply to cer- tain electronic transitions andnot toothers..
Correspondence between Classical and Quantum Mechanical
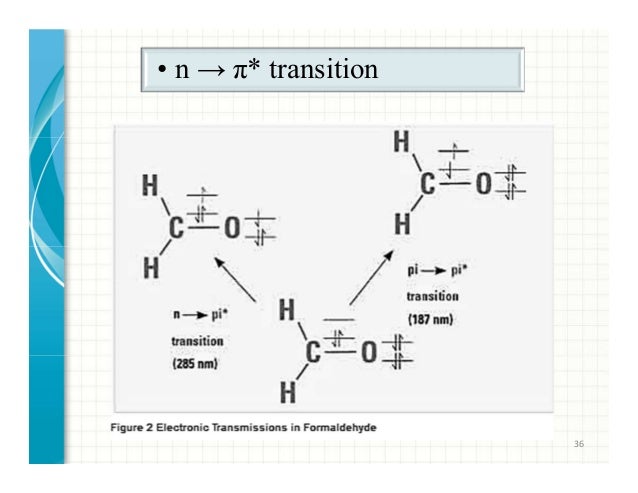
Electronic Transitions University of Texas at Dallas. Selection rules for electronic transitions The Beer-Lambert Law A = log 10 (I o/I) = εcl where ε is the molar extinction coefficient ( in L cm-1 mole-1), c is concentration in mole The rules above for electronic transitions are of only limited use in interpreting spectra, because one is very rarely concerned with purely electronic transitions..
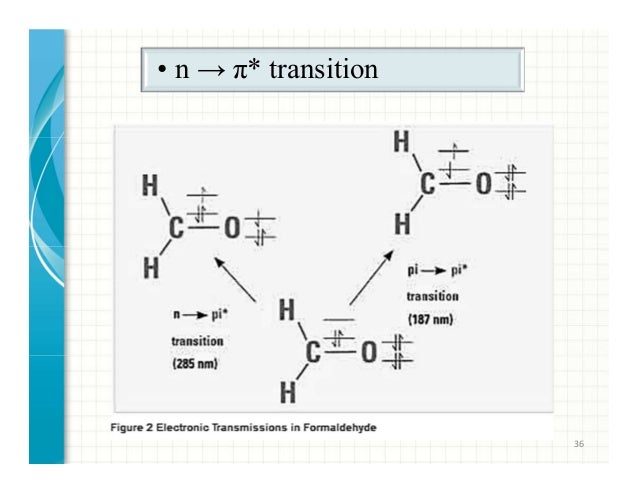
Selection rules • The outer shell electrons define – a total orbital angular momentum L=Σl i, – a total spin angular momentum S and (In modern parlance, we are ``deriving'' the selection rule for electric dipole transitions, and ignoring magnetic dipole transitions.) The uniform electric field can shove the electron this way or that, but cannot flip it over.
4.1.2 Polarization selection rules 19 4.1.3 Vibronic transitions 21 4.2 Allowed electric and magnetic dipole transitions 21 4.3 One photon transitions within the 4fN configuration 21 3. 4 Contents 4.3.1 General theory 21 4.3.2 Transitions between crystal-field levels 22 4.3.3 Transitions between J multiplets 22 5 Intensity Examples 24 5.1 Ce3+ examples 24 5.1.1 Atomic calculation exercise 24 The quantum-mechanical selection rules for electric dipole radiative transitions between atomic energy levels are derived, firstly for one-electron atoms without spin, and then including spin angular momentum. The discussion is extended to many-electron atoms and rules for L, S, and J quantum numbers are derived.
Selection rules 2. Laporte selection rule: there must be a change in the parity (symmetry) of the complex Electric dipole transition can occur only between states of opposite parity. The rules above for electronic transitions are of only limited use in interpreting spectra, because one is very rarely concerned with purely electronic transitions.
PDF We report on the polarization selection rules of inter-Landau-level transitions using reflection-type optical Hall effect measurements from 600 to 4000 cm^{-1} on epitaxial graphene grown by Selection Rules for Electronic Transitions In spectral phenomena such as the Zeeman effect it becomes evident that transitions are not observed between all pairs of energy levels. Some transitions are "forbidden" ( i.e., highly improbable) while others are "allowed" by a set of selection rules.
electronic band by considering that the electronic transition takes place faster than the change of the nuclear configuration in the excited state of the molecule. The above equation is of great importance because the first integral defines the electronic selection rules, while the second integral is the basis of vibrational selection rules.
If we consider the rotational states as well, it is required that the total angular momentum of photon and molecule remains constant. In general, the selection rules for the total angular momentum are as follows: however, for transitions the transition is forbidden. be considered for electronic transitions, called “selection rules” ’ (1) The spin quantum number of an electron should not change during the electronic
If we consider the rotational states as well, it is required that the total angular momentum of photon and molecule remains constant. In general, the selection rules for the total angular momentum are as follows: however, for transitions the transition is forbidden. 3 Selection Rules • During the transition, there must be a change in the dipole moment of the molecule: ¾if there is a large change, the light / molecule interaction is strong and
The selection rules for magnetic dipole transitions follow from equation (3.5): only transi- tions between states in which the matrix elements of either L or S are nonzero are magnetic- dipole allowed. Section 2 Atomic Spectra (Lectures 2-3ish) Atomic Structure: IC yr 1 Quantum theory of atoms / molecules Previously: H-Atom: Wavefunctions, quantum numbers and energy levels Spectral transitions: Rydberg series and selection rules Spin and spin-orbit coupling Alkali Metal Atoms (e.g., Na): Penetration and shielding, quantum defects Larger spin-orbit coupling He Atom Singlets and …
Electronic Spectroscopy Application of Group Theory • Ψ Tot assumed to be separable • If a transition is not allowed by symmetry then vibronic I. Rotational Transitions Within the approximation that the electronic, vibrational, and rotational states of a molecule can be treated as independent, the total molecular wavefunction of the "initial" state is a product Φ i = ψ ei χ vi φri of an electronic function ψ ei, a vibrational function χ vi, and a rotational function φri. A similar product expression holds for the "final
10.12.2004 1 Electronic Transitions Uni Siegen Physikalische Chemie Ø Selection rules Ø Transition strength Ø Franck-Condon-Factor S1 S0 T1 ISC Jablonski Electric Dipole Approximation and Selection Rules We can now expand the term to allow us to compute matrix elements more easily. Since and the matrix element is squared, our expansion will be in powers of which is a small number.
Electric Dipole Approximation and Selection Rules We can now expand the term to allow us to compute matrix elements more easily. Since and the matrix element is squared, our expansion will be in powers of which is a small number. 7/06/2013 · The apparent preservation of the parity selection rule indicates that the π-conjugation structure, which is mostly responsible for optical electronic transitions in this system, still remains quasi-symmetrical in H 2 TBTAC.