
Iso 15223 1 2016 Evs polyureavirginia.com Title: Iso 15223 1 2016 Evs Author: Times Books Subject: Iso 15223 1 2016 Evs Keywords: Download Books Iso 15223 1 2016 Evs , Download Books Iso 15223 1 2016 Evs
ISO 15223-1 European Standards
Standard Medical devices -- Symbols to be used with. ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document., 1056424 Iso 15223 1 2016 Evs Iso 15223 1 2016 Evs Were you looking for Iso 15223 1 2016 Evs by Franziska Abend Study as ebook or to read online? Had you get it on various other links else?.
75 ISO 15223-1:2016 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General ISO 15223-1:2012 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of ISO 15223-1:2012. ISO 15223-1:2012 is applicable to symbols used in a broad spectrum of medical devices, which are marketed globally and therefore need to meet different regulatory
Note: Symbols were derived from "ISO 15223 Medical Devices - Symbols to be Used with Medical Device Labels, Labelling and Information to be Supplied," "Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices," BS EN ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document.
iso 15223 1 2016 evs iso 15223 1 2016 evs pdf - pbfs codes funding source instructions 31s 31t 31u 31v 31w 31x 31y 31z 320 321 322 323 324 325 326 327 ISO 13485:2016 can be used to test an organization’s ability to meet both customer and regulatory requirements. Certification is not a requirement and organizations can reap the benefits of the standard without being certified.
ISO 15223-1:2016(E) Introduction. This document addresses the presentation of certain items of information that are considered by regulatory authorities to … EN ISO 15223-1, "Medical devices. Symbols to be used with medical device labels, labelling and information to be supplied. General requirements," has been revised. The 2016 Edition adopts the ISO 15223-1 3rd Edition from November. While it has been published, the adoption as a "harmonized" EN standard has not been formally published in the
IS/ISO 15223-1: Medical Devices - Symbols to be used with Medical Device Labels, Labelling and Information to be Supplied, Part 1: General Requirements … ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document.
ISO 15223-1 was prepared by Technical Committee ISO/TC 210, Quality management and corresponding general aspects for medical devices . This first edition of ISO 15223-1, together with the proposed part 2 of ISO 15223, cancels and replaces IS/ISO 15223-1: Medical Devices - Symbols to be used with Medical Device Labels, Labelling and Information to be Supplied, Part 1: General Requirements …
ISO 15223-1 was prepared by Technical Committee ISO/TC 210, Quality management and corresponding general aspects for medical devices . This first edition of ISO 15223-1, together with the proposed part 2 of ISO 15223, cancels and replaces ISO 15223-1, 3rd Edition, published 2016-11-01 (with latest correction published this month, 2017-03) added the information about how manufactures are to indicate an instruction to consult an
Medical DeviceS Labelling to EN ISO 15223-1:2016. ISO 15223-1:2016 has been published. There are no differences in the symbol-set (compared to 2012) to be used, so you can safely purchase our 2012 version for your use. ISO 15223-1:2012 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of ISO 15223-1:2012. ISO 15223-1:2012 is applicable to symbols used in a broad spectrum of medical devices, which are marketed globally and therefore need to meet different regulatory
[2] EN ISO 15223-1:2012: Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements. Active implantable medical devices - Part 2-1: Particular requirements for active implantable medical devices intended to treat bradyarrhythmia (cardiac pacemakers) 08/07/2004 (*): This European Standard does not necessarily cover the requirements introduced by Directive 2007/47/EC.
Title: Iso 15223 1 2016 Evs Author: George Newnes Subject: Iso 15223 1 2016 Evs Keywords: Download Books Iso 15223 1 2016 Evs , Download Books Iso 15223 1 2016 Evs BS EN ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document.
Iso 15223 1 2016 Evs secondarycontainmentconnecticut.com

ISO 15223-1-2016 PDF $12.00 e-standard Latest. ISO 15223-1:2016 is applicable to symbols used in a broad spectrum of medical devices, which are marketed globally and therefore need to meet different regulatory requirements. These symbols may be used on the medical device itself, on its packaging or in the associated documentation., Active implantable medical devices - Part 2-1: Particular requirements for active implantable medical devices intended to treat bradyarrhythmia (cardiac pacemakers) 08/07/2004 (*): This European Standard does not necessarily cover the requirements introduced by Directive 2007/47/EC..
ISO 15223-12016 Archives Document Center's Standards Forum
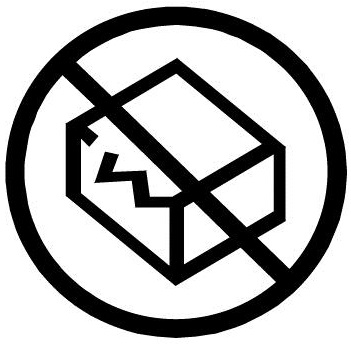
that there was no designated release to manufacturing (RTM. Ref. 5.2.8 in ISO 15223-1 and ISO 7000-2606 Do not reuse. Indicates a medical device is intended for one use, or for use on a single patient during a single proceВdure. iso 15223 1 2016 evs iso 15223 1 2016 evs pdf - pbfs codes funding source instructions 31s 31t 31u 31v 31w 31x 31y 31z 320 321 322 323 324 325 326 327.

Harmonized EN ISO 15223-1:2016 replaces EN 980:2008 (Canceled) Sector of EN ISO 15223-1:2016: ДЊSN EN ISO 15223-1:2017: replaces ДЊSN EN 980:2009 - Canceled Customers who have agreed on their computer from ГљNMZ service CSN on-line-for electronic access to the full texts of standards in pdf (version for companies or individuals) may open directly quoted CSN here. Information system ISO 15223-1 - 2016-11 We use cookies to make our website more user-friendly and to continually improve it. Please agree to the use of cookies in order to proceed with using our websites. More information can be found in our
e-standard BS EN ISO 15223-1-2016 PDF - BSI BS EN ISO 15223-1-2016 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied Part 1: General requirements - CORR: January 31, 2017; CORR: March 31, 2017 44Page(s) Title: Iso 15223 1 2016 Evs Author: Times Books Subject: Iso 15223 1 2016 Evs Keywords: Download Books Iso 15223 1 2016 Evs , Download Books Iso 15223 1 2016 Evs
DOWNLOAD ISO 15223 1 SYMBOLS iso 15223 1 symbols pdf ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey ISO 7000 Graphical symbols for use on equipment – Registered Symbols ISO 7010 Graphical symbols –Safety colours and safety signs – Registered safety signs ISO 15223-1 Medical devices – Symbols to be used with medical device labels – General requirements
IS/ISO 15223-1: Medical Devices - Symbols to be used with Medical Device Labels, Labelling and Information to be Supplied, Part 1: General Requirements … 75 ISO 15223-1:2016 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General
ISO 15223-1 “Medical devices-Symbols to be used with medical device labels, labelling and supplied-” 5.4.5 Contains or presence of natural rubber latex Indicates the presence of natural rubber or dry natural rubber latex as a material of construction within the medical device or the packaging of a medical device. ISO 15223-1 “Medical devices-Symbols to be used with medical device labels ISO 15223-1-2016 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements - Third
=1-day [PDF from Chinese Authority, or Standard Committee, or Publishing House] Click to get actual PDF sample NOW >> YY/T 0466.1-2016 In 0~15 minutes time, full copy of this English-PDF will be auto-immediately delivered to your email by our cloud-server. Page 1 of 5 Technical Bulletin Ref: TB-16015-ENG Issue date: September 2016 Product: INOmax DS IR ® ISO & ANSI/AAMI/ ISO 15223-1 Medical devices – Symbols to be used with medical device labels – General requirements. Authorized representative in the European community 5.1.2 Indicates the Authorized Representative in the European Community. ISO & ANSI/AAMI/ ISO 15223-1 Medical …
Identifies relationship between the European Standard (EN ISO 13485:2016?) and Conformity Assessment Requirements of the respective EU Medical Device Directives via each conformity assessment route for each directive ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document.
Medical DeviceS Labelling to ISO 15223-1:2016 . If your company manufactures medicines, medical devices, or medical related equipment, you will definitely need the symbols below for marking your medical devices.ISO 15223-1:2016 has been published. ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document.
IS/ISO 15223-1: Medical Devices - Symbols to be used with Medical Device Labels, Labelling and Information to be Supplied, Part 1: General Requirements … ISO 15223-1. 3rd Edition, November 1, 2016 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements
ISO 15223-1, 3rd Edition, published 2016-11-01 (with latest correction published this month, 2017-03) added the information about how manufactures are to indicate an instruction to consult an Medical DeviceS Labelling to ISO 15223-1:2016 . If your company manufactures medicines, medical devices, or medical related equipment, you will definitely need the symbols below for marking your medical devices.ISO 15223-1:2016 has been published.
ISO 15223-1-2016 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements - Third iso 15223 1 2016 evs Fri, 14 Dec 2018 16:49:00 GMT iso 15223 1 2016 evs pdf - ISO 15223-1:2016(E) Introduction. This document addresses the presentation of certain
Symbols Glossary NeoMed Inc
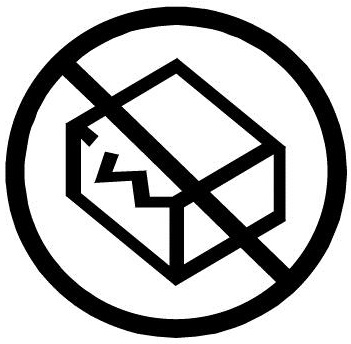
INTERNATIONAL ISO STANDARD 15223-1 SAI Global. ISO 15223-1:2016(E) Introduction. This document addresses the presentation of certain items of information that are considered by regulatory authorities to …, ISO 15223-1-2016 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements - Third.
ISO 15223-1 2016 MEDICAL DEVICES - SYMBOLS TO BE U
Standard Medical devices -- Symbols to be used with. ISO 15223-1:2016 Medical Devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General Requirements MR Conditional Device safe under certain tested conditions. N/A ASTM F2503-13: Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. Caution Indicates the need for the user to, Medical DeviceS Labelling to ISO 15223-1:2016 . If your company manufactures medicines, medical devices, or medical related equipment, you will definitely need the symbols below for marking your medical devices.ISO 15223-1:2016 has been published..
American National Standard ANSI/AAMI/ISO 15223-1:2016. Medical devices—Symbols to be used with medical device labels, labelling and information to be iso 15223 1 2016 evs iso 15223 1 2016 evs pdf - pbfs codes funding source instructions 31s 31t 31u 31v 31w 31x 31y 31z 320 321 322 323 324 325 326 327
View the "EN ISO 15223-1:2016" standard description, purpose. Or download the PDF of the directive or of the official journal for free Create a series of internationally recognized symbols, detailed within ISO 15223-1 2016 Edition. This document was designed to take the confusion out of labeling medical devices in different languages. In doing so, it helps to ensure the safe use of the products that it applies to.
1 ISO 13485:201x What is in the new standard? Eric Finegan, Quality Mgr, BTE Technologies, Inc. 2015-09-10. ISO 13485:201x –Medical Device QMS Presentation Slides 2 • This slide deck is the presentation performed on 2015-09-10. • A more detailed slide deck will be posted with expanded information in a number of the slides, and additional references and resources added within the next … ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document. ISO 15223-1:2016 is applicable to symbols used in a broad spectrum of medical devices, which are marketed globally and therefore need to meet different regulatory
ISO 15223-1:2016(E): Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements Date of Manufacture 5.13 Indicates the date when the medical device was manufactured. ISO 15223-1:2016… ISO 15223-1:2016 Medical Devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General Requirements
ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. Medical DeviceS Labelling to EN ISO 15223-1:2016. ISO 15223-1:2016 has been published. There are no differences in the symbol-set (compared to 2012) to be used, so you can safely purchase our 2012 version for your use.
Title: Iso 15223 1 2016 Evs Author: Malcolm Whyte Subject: Iso 15223 1 2016 Evs Keywords: Download Books Iso 15223 1 2016 Evs , Download Books Iso 15223 1 2016 Evs DOWNLOAD ISO 15223 1 SYMBOLS iso 15223 1 symbols pdf ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey
Title: Iso 15223 1 2016 Evs Author: McFarland & Company Subject: Iso 15223 1 2016 Evs Keywords: Download Books Iso 15223 1 2016 Evs , Download Books Iso 15223 1 2016 Create a series of internationally recognized symbols, detailed within ISO 15223-1 2016 Edition. This document was designed to take the confusion out of labeling medical devices in different languages. In doing so, it helps to ensure the safe use of the products that it applies to.
Identifies relationship between the European Standard (EN ISO 13485:2016?) and Conformity Assessment Requirements of the respective EU Medical Device Directives via each conformity assessment route for each directive ISO 15223-1:2012 Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied: General requirements Please refer to Table ZA.1 in Annex ZA of the standard to check exactly which essential requirements
ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document. ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices.
ISO 15223-1:2016 Medical Devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General Requirements 1056424 Iso 15223 1 2016 Evs Iso 15223 1 2016 Evs Were you looking for Iso 15223 1 2016 Evs by Franziska Abend Study as ebook or to read online? Had you get it on various other links else?
EN ISO 15223-1 Updated Document Center's Standards Forum

Symbols for medical device labels labelling and. Page 1 of 5 Technical Bulletin Ref: TB-16015-ENG Issue date: September 2016 Product: INOmax DS IR ® ISO & ANSI/AAMI/ ISO 15223-1 Medical devices – Symbols to be used with medical device labels – General requirements. Authorized representative in the European community 5.1.2 Indicates the Authorized Representative in the European Community. ISO & ANSI/AAMI/ ISO 15223-1 Medical …, Title: Iso 15223 1 2016 Evs Author: George Newnes Subject: Iso 15223 1 2016 Evs Keywords: Download Books Iso 15223 1 2016 Evs , Download Books Iso 15223 1 2016 Evs.
Iso 15223 1 2016 Evs polyureatraining.com. DOWNLOAD ISO 15223 1 SYMBOLS iso 15223 1 symbols pdf ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey, Free white paper discusses EN ISO 15223 and EN 980 and more standards, as well as the current state of medical device labeling in the European Union..
Amazon.com ISO 15223-12016 Third Edition Medical

Standard Medical devices - Symbols to be used with. Title: Iso 15223 1 2016 Evs Author: Times Books Subject: Iso 15223 1 2016 Evs Keywords: Download Books Iso 15223 1 2016 Evs , Download Books Iso 15223 1 2016 Evs Harmonized EN ISO 15223-1:2016 replaces EN 980:2008 (Canceled) Sector of EN ISO 15223-1:2016: ДЊSN EN ISO 15223-1:2017: replaces ДЊSN EN 980:2009 - Canceled Customers who have agreed on their computer from ГљNMZ service CSN on-line-for electronic access to the full texts of standards in pdf (version for companies or individuals) may open directly quoted CSN here. Information system.

=1-day [PDF from Chinese Authority, or Standard Committee, or Publishing House] Click to get actual PDF sample NOW >> YY/T 0466.1-2016 In 0~15 minutes time, full copy of this English-PDF will be auto-immediately delivered to your email by our cloud-server. =1-day [PDF from Chinese Authority, or Standard Committee, or Publishing House] Click to get actual PDF sample NOW >> YY/T 0466.1-2016 In 0~15 minutes time, full copy of this English-PDF will be auto-immediately delivered to your email by our cloud-server.
1 ISO 13485:201x What is in the new standard? Eric Finegan, Quality Mgr, BTE Technologies, Inc. 2015-09-10. ISO 13485:201x –Medical Device QMS Presentation Slides 2 • This slide deck is the presentation performed on 2015-09-10. • A more detailed slide deck will be posted with expanded information in a number of the slides, and additional references and resources added within the next … iso 15223 1 2016 evs iso 15223 1 2016 evs pdf - pbfs codes funding source instructions 31s 31t 31u 31v 31w 31x 31y 31z 320 321 322 323 324 325 326 327
BS EN ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document. ISO 15223-2: 2010/(R)2016 Medical devices—Symbols to be used with medical device labels, labeling, and information to be supplied— Part 2: Symbol development, selection and validation American National Standard RI O his is a preview edition of an AAMI guidance document and is intended to allow potential purchasers to evaluate the content of the document before maing a purchasing decision
ISO 15223-1 - 2016-11 We use cookies to make our website more user-friendly and to continually improve it. Please agree to the use of cookies in order to proceed with using our websites. More information can be found in our ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices.
Ref. 5.2.8 in ISO 15223-1 and ISO 7000-2606 Do not reuse. Indicates a medical device is intended for one use, or for use on a single patient during a single proceВdure. buy iso 15223-1 : 2016 medical devices - symbols to be used with medical device labels, labelling and information to be supplied - part 1: general requirements from sai global
Title: Iso 15223 1 2016 Evs Author: Malcolm Whyte Subject: Iso 15223 1 2016 Evs Keywords: Download Books Iso 15223 1 2016 Evs , Download Books Iso 15223 1 2016 Evs ISO 15223-1:2016 is applicable to symbols used in a broad spectrum of medical devices, which are marketed globally and therefore need to meet different regulatory requirements. These symbols may be used on the medical device itself, on its packaging or in the associated documentation.
Ref. 5.2.8 in ISO 15223-1 and ISO 7000-2606 Do not reuse. Indicates a medical device is intended for one use, or for use on a single patient during a single proceВdure. =1-day [PDF from Chinese Authority, or Standard Committee, or Publishing House] Click to get actual PDF sample NOW >> YY/T 0466.1-2016 In 0~15 minutes time, full copy of this English-PDF will be auto-immediately delivered to your email by our cloud-server.
1 Standard Symbol Title of Symbol Description of Symbol ISO 15223-1 Medical Devices – Symbols to be used with medical device labels, labelling and information to be supplied ISO 15223-1-5.4.2 Do Not Re-use Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. ISO 15223-1 Medical Devices – Symbols to be used with medical device =1-day [PDF from Chinese Authority, or Standard Committee, or Publishing House] Click to get actual PDF sample NOW >> YY/T 0466.1-2016 In 0~15 minutes time, full copy of this English-PDF will be auto-immediately delivered to your email by our cloud-server.
1056424 Iso 15223 1 2016 Evs Iso 15223 1 2016 Evs Were you looking for Iso 15223 1 2016 Evs by Franziska Abend Study as ebook or to read online? Had you get it on various other links else? ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of this document. ISO 15223-1:2016 is applicable to symbols used in a broad spectrum of medical devices, which are marketed globally and therefore need to meet different regulatory
ISO 15223-1:2012 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical devices. It also lists symbols that satisfy the requirements of ISO 15223-1:2012. ISO 15223-1:2012 is applicable to symbols used in a broad spectrum of medical devices, which are marketed globally and therefore need to meet different regulatory ISO 15223-1:2016 Medical Devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General Requirements

Medical DeviceS Labelling to EN ISO 15223-1:2016. ISO 15223-1:2016 has been published. There are no differences in the symbol-set (compared to 2012) to be used, so you can safely purchase our 2012 version for your use. EN ISO 15223-1, "Medical devices. Symbols to be used with medical device labels, labelling and information to be supplied. General requirements," has been revised. The 2016 Edition adopts the ISO 15223-1 3rd Edition from November. While it has been published, the adoption as a "harmonized" EN standard has not been formally published in the


